Recombinant SARS-CoV-2, S1 Subunit Protein (RBD)
Recombinant SARS-CoV-2, S1 Subunit Protein (RBD) with C-terminal His tag, derived from the transfected human HEK293 cells.
Product Description
Specifications
| Size | 100 ug, 500 ug, 1000 ug |
|---|---|
| Species | SARS-CoV-2 |
| Accession Number | QHD43416 |
| Protein Name / Synonyms | Spike protein, S Protein, S1 Subunit, Host Cell Receptor Binding Domain (RBD) |
| Expressed Region | Arg319-Phe541 |
| Expression System | HEK293 cells |
| Tag | C-terminal his-tag |
| Purification | His-tag affinity purification by immobilized metal ion affinity chromatography (IMAC) |
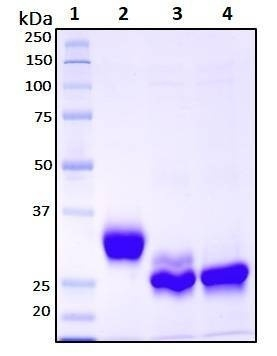
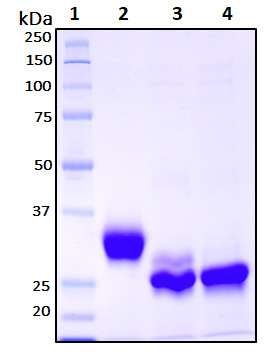
| Purity | >95 % |
| Purity Determined By | SDS-PAGE under reducing conditions and visualized by Coomassie blue staining |
| Molecular Weight (kDa) | Recombinant protein product has a calculated molecular mass of 25 kDa. Due to the abundant glycosylation, it migrates as approximately 30 kDa protein bands in SDS-PAGE under DTT, beta-mercaptoethanol reducing conditions. See deglycosylation analysis image below. |
| Format | Liquid |
| Formulation | Supplied as a 0.2 µm filtered solution in PBS (pH 7.4) |
| Concentration (lot specific) | Lot specific (see the label on the vial), determined by BCA protein assay |
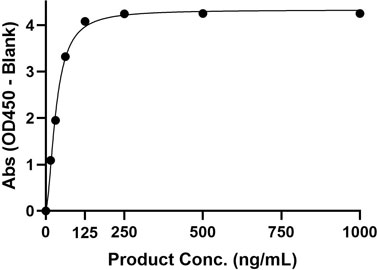
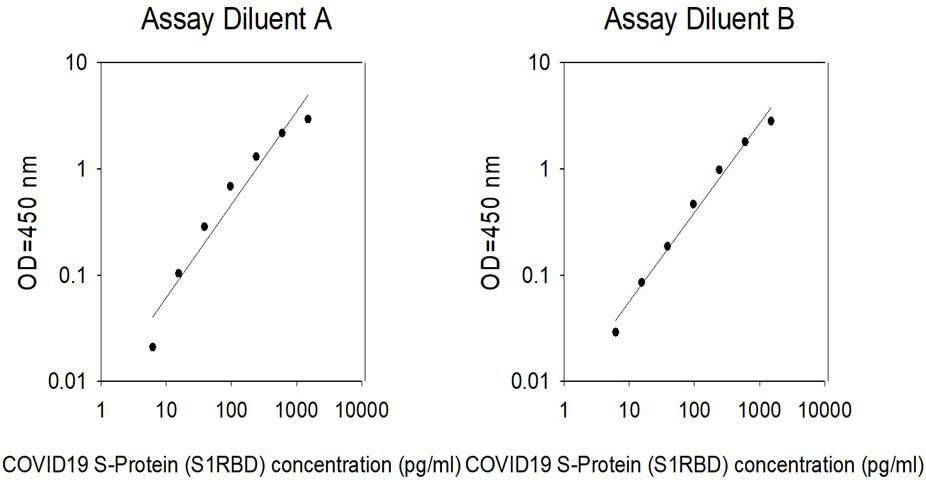
| Research Area | Antibody Drug Development, Receptor-Ligand Binding |
| Estimated Lead Time | 1-2 business days |
| Shipping Type | Blue ice |
| Storage | -20°C |
A Antonopoulos, et al. Site-specific characterisation of SARS-CoV-2 spike glyocprotein receptor binding domain. Glycobiology, cwaa085 (2020).
C. D. Camell et al., Science 373, eabe4832 (2021). DOI: 10.1126/science.abe4832
Beltr?n-Pavez C, et al. Insights into neutralizing antibody responses in individuals exposed to SARS-CoV-2 in Chile. Science Advances (2021).
Borchers C, et al. Evaluation of SARS-CoV-2 spike S1 protein response on PI3K-mediated IL-8 release. Med Sci (Basel) 18;9(2):30 (2021).
Keshavarz B, et al. Quantitative measurement of IgG to severe acute respiratory syndrome coronavirus-2 proteins using immunoCAP. Int Arch Allergy Immunol. 182(5):417-424 (2021).
Miller CJ, et al. FN3-based monobodies selective for the receptor binding domain of the SARS-CoV-2 spike protein. N Biotechnol (2021).
Pirovano, G., Ordonez, A. A., Jain, S. K., Reiner, T., Carroll, L. S., & Pillarsetty, N. V. K. (2021). Rapid detection of SARS-CoV-2 using a radiolabeled antibody. Nuclear Medicine and Biology, 98-99, 69?75. doi:10.1016/j.nucmedbio.2021.05.0
Luetkens T, Metcalf R, Planelles V, Zheng Y, Larragoite ET, Spivak ES, Spivak AM, Steinbach M, Blaylock RC, Avila SV, Hankey KG, Martins TB, Slev PR, Mannuel HD, Sajadi M, Rapoport AP, Atanackovic D. Successful transfer of anti-SARS-CoV-2 immunity using convalescent plasma in an MM patient with hypogammaglobulinemia and COVID-19. Blood Adv. 2020 Oct 13;4(19):4864-4868. doi: 10.1182/bloodadvances.2020002595. PMID: 33031540; PMCID: PMC7556131.
Species:
Human
Sample Type:
Plasma
Huang W-C., Zhou S., He X., et al. SARS-CoV-2 RBD Neutralizing Antibody Induction is Enhanced by Particulate Vaccination. Adv. Mater. 2020, 2005637
Species:
Human
Sample Type:
Conditioned Media (Liposomes coated with RBD antigen)
-
Great productWe emploied this protein for site-specific glycosylation analysis. It works great.
from University of California, Davis,
on
Write Your Own Review
 Datasheet
Datasheet