Stable Cell Line Generation
RayBiotech’s stable cell line generation service uses a mammalian retrovirus to incorporate foreign genes into the host genome. This enables the gene to be passed to daughter cells and maintained without the use of antibiotics. Our comprehensive service cover every step of the workflow, from gene synthesis to cell culture and treatment.
Quick Links:
Why You Should Choose Our Stable Cell Line Development Services
- Comprehensive— We can help you with everything from gene synthesis to cell culture and treatment. Our services include data analysis.
- Customizable— The project is tailored to your experimental needs. Choose which phases are needed and RayBiotech or you can supply the materials.
- Quality— GMP compliant, GLP compliant, ISO 13485 certified.
- Trust— Trusted biotechnology company since 2001 with many success stories. Contact us for examples!
- Support— Responsive & knowledgeable technical support team with real lab experience. We can help guide your experimental design.
- Affordable
What are the advantages of stable gene expression?
Compared to transient gene expression, stable expression has the following advantages:
- Gene will be passed to daughter cells
- Cultured cells are genetically identical
- Large amounts of the protein-of-interest can be produced
- No antibiotic selection is required to maintain gene expression
What are the potential applications of stable gene expression?
- Functional studies (of a specific protein)
- Drug screening
- Production of therapeutic proteins
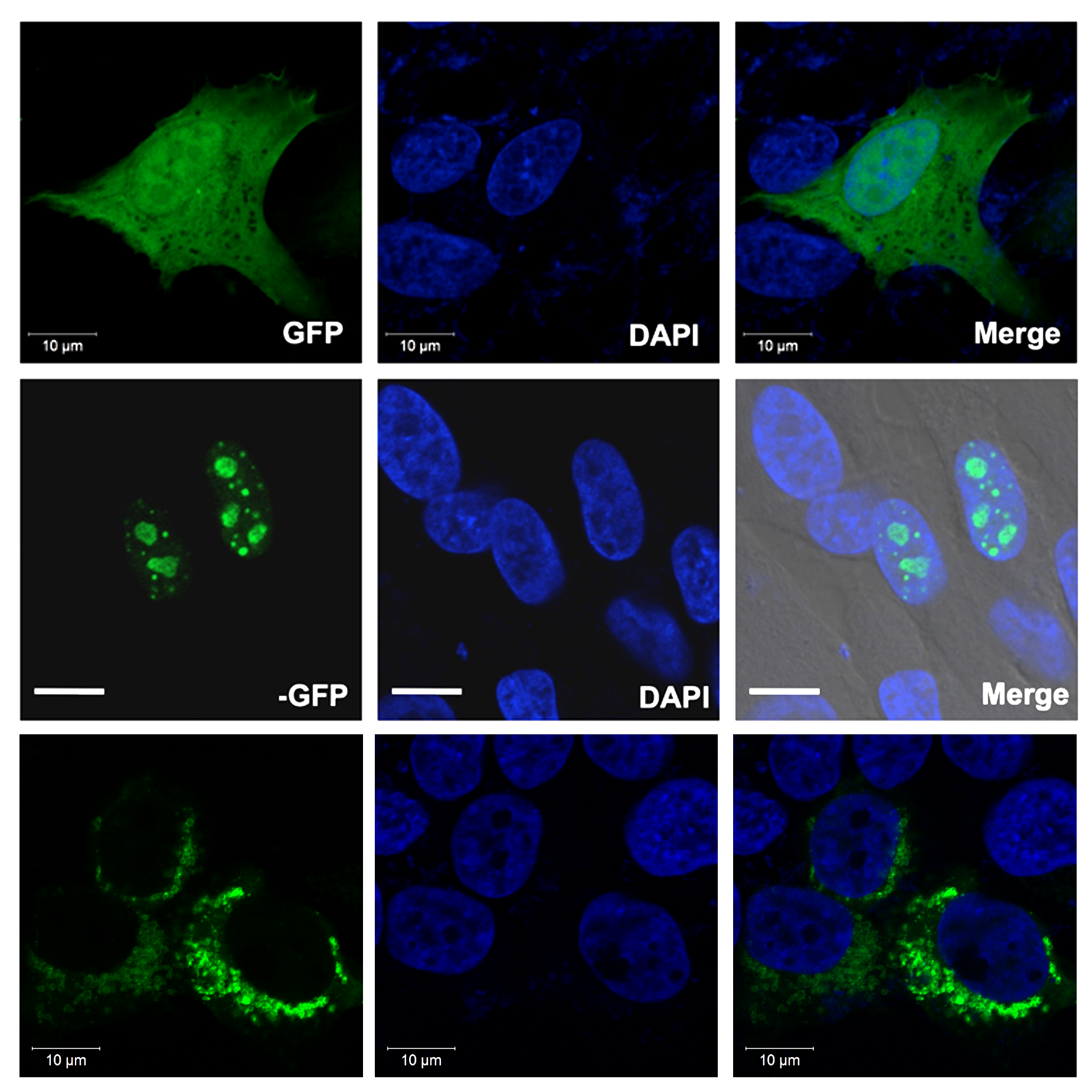
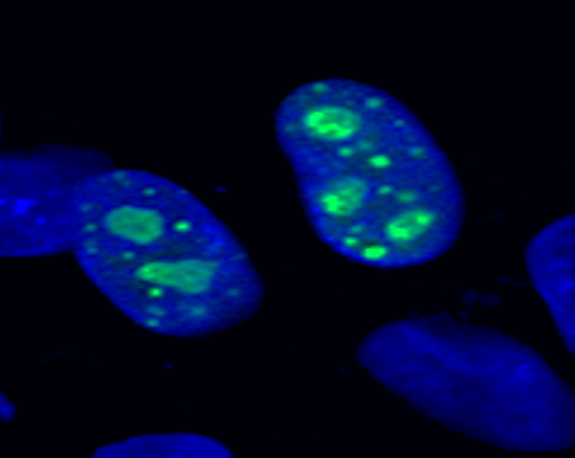
Protein localization of the foreign gene is visualized via the GFP fusion tag. Cell images using a A) GFP filter or B) DAPI filter. C) A and B images merged. 1) Only GFP was expressed; GFP did not localize to a specific cellular component. 2) GeneX-GFP localized to nucleoli within the nucleus. White bar = 10 µm. 3) GeneY-GFP localized to mitochondria within the cytoplasm.
See What Our Stable Cell Line Service Can Do For Your Research
How Our Stable Cell Line Generation Service Works
Phase 1: Gene synthesis (usually 2-4 weeks)
The target gene is synthesized.
Phase 2: Gene cloning (2 weeks)
The gene-of-interest will be cloned into a retrovirus plasmid. Proper insertion of the gene is confirmed via DNA sequencing.
Phase 3: Retroviral packaging (2 weeks)
The retrovirus titer is determined using human HEK293 cells. Options for shipping include:
- Mini-scale production (3-5 ml of virus; concentrated viral stock not available)
- Standard production (200 µL concentrated viral stock; ~1E8 to ~1E9 pfu/ml)
- Large-scale production (1 ml concentrated viral stock; ~1E8 to ~1E9 pfu/ml)
Phase 4: Retrovirus titering (2-3 weeks)
The retrovirus titer is determined using human HEK293 cells.
Phase 5: Stable cell line generation (1-2 months)
Cells are transduced with the gene-of-interest. Stable gene expression will be confirmed with a western blot.
Phase 6: Cell culture & treatment (depends on experiment)
We can culture and treat the stable cells according to your specifications. Data analysis included.