PK/PD Services
Pharmacokinetic & Pharmacodynamic Analysis
RayBiotech offers expert profiling services for your therapeutic antibodies, providing valuable insights into the drug's behavior in the body through pharmacokinetic (PK) and pharmacodynamic (PD) analyses. Our multi-step approach enables a deeper understanding of the drug's pharmacological characteristics, enhancing your confidence in its efficacy and safety.
- Quantification of drug metabolites in biological samples
- Quantitative dose-response for optimization of drug efficiency
- Time-course and dose-effect to target cells
- Safety analysis of dose-dependent host response
- EC50
- Receptor occupancy analysis including free receptor, occupied receptor, total receptor assays
- Cell toxicity
- Mechanism of action
Pharmacokinetics (PK)
Drug Pharmacokinetics studies revolve around how the organism affects the drug, and these studies for antibody and small molecule drug are critical to any drug design. How much drug is absorbed into the system, how wide, and where the distribution is, as well as how long the drug is maintained in the system are common questions about any treatments. Other questions is what the drug is doing to the immune system in vivo, with testing of drug immunogenicity, either via anti drug antibody, or other immune interactions being a critical piece of information.
RayBiotech offers many options to test in vitro samples, as well as serum and plasma drug levels in your samples to measure the interactions ongoing. Our ELISA Drug antibody and anti-drug antibody ELISA and RayPlex kits are available for a number of widely tested drugs. Additionally, if you need help or technical expertise, we offer consulting options as well as full testing options to help where needed.
Standard ELISA and bead-based Flow Cytometry Kits (RayPlex) available for:
- Drug Absorption Rate
- Drug Distribution
- Drug Elimination
- Drug Immunogenicity
Drug PK Services Available
| Parameters | Test and Assay Options |
|---|---|
| Absorption rate | Plasma Antibody Drug levels – Standard ELISA, Flow-based RayPlex |
| Drug distribution | Plasma Antibody Drug levels – Standard ELISA, Flow-based RayPlex |
| Elimination (half-life) | Plasma Antibody Drug levels – Standard ELISA, Flow-based RayPlex |
| Immunogenicity studies | Anti-drug antibody (ADA) testing - Standard ELISA, Flow-based RayPlex |
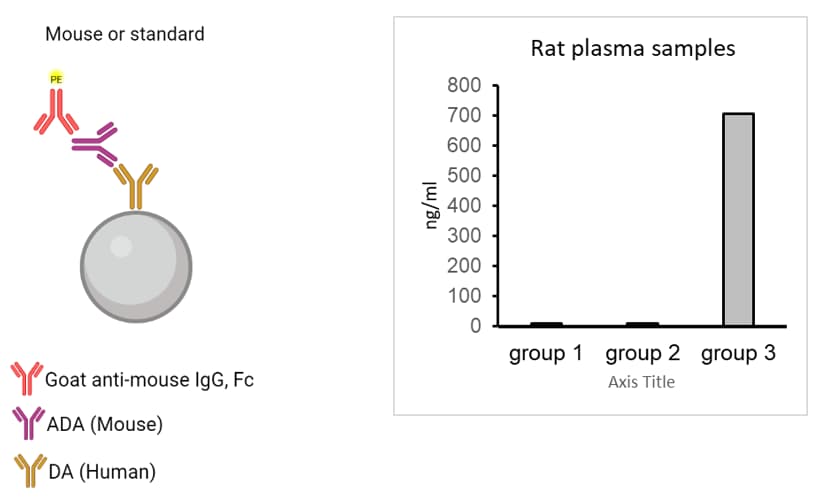
RayBiotech’s Method of Drug Antibody and Anti-Drug Antibody Assays
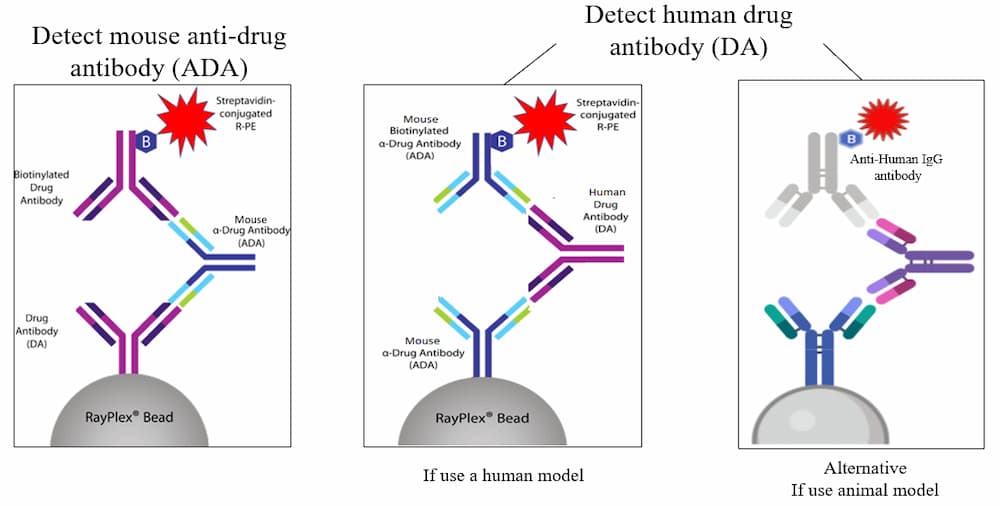
Drug PK Kits – Both DA & ADA kits available with RayPlex® Bead Arrays
| Target | Drug Antibody (DA) | Anti Drug Antibody (ADA) | ADA species |
|---|---|---|---|
| PD-I | Nivol umab/Opdivo | Anti-Nivolumab Antibody | mAb, Mouse |
| PD-I | Pembrolizumab/Keytruda | Anti-Pembrolizumab Antibody | mAb, Mouse |
| PD-LI | Atezolizumab/Tecentriq | Anti-Atezolizumab Antibody | mAb, Mouse |
| PD-LI | Avelumab/Bavencio | Anti-Avelumab Antibody | mAb, Mouse |
| CTLA-4 | Ipilimumab/Yervoy | Anti-Ipilimumab | mAb, Mouse |
| HER2 | Pertuzumab/Perjeta | Anti-Pertuzumab | mAb, Mouse |
| HER2 | Trastuzumab/Herceptin | Anti -Trastuzumab Antibody | mAb, Mouse |
| TNF-α | Adalimumab/Humira | Anti-Adalimumab Antibody | mAb, Mouse |
| TNF-α | Etane rcept/Enbrel | Anti -Etanercept Antibody | mAb, Mouse |
| TNF-α | Infliximab/Remicade | Anti-InfIiximab Antibody | mAb, Mouse |
| RANK ligand | Denosumab/Prolia | Anti-Denosumab | mAb, Mouse |
| EGF receptor | Cetuximab/Erbitux | Anti -Cetuximab | mAb, Mouse |
| EGF receptor | Panitumumab/Vectibix | Anti-panitumumab | mAb, Mouse |
| VEGE-A | Bevacizumab/Avastin | Anti-Bevacizumab | mAb, Mouse |
| VEGF-A | Ranibizumab/Lucent is | Anti-Ranibizumab | mAb, Mouse |
| Complement protein C5 | Eculizumab/Soliris | Anti-Eculizumab | mAb, Mouse |
| CD20 | Obinutuzumab/Gazyva | Anti -Obinutuzumab Antibody | mAb, Mouse |
| CD20 | Rituximab/Rituxan | Anti -Rituximab Antibody | mAb, Mouse |
| CD274 | Durvalumab/lmfinzi | Anti -Durvalurnab Anti body | mAb, Mouse |
| CD38 | Daratumumab/Darzalex | Anti-Daratumumab Antibody | mAb, Mouse |
| Human lgE | Omalizumab/Xolair | Anti-Omalizumab | mAb, Mouse |
| IL-12 and IL-23 | Ustekinumab/Stelara | Anti -Ustekinumab | mAb, Mouse |
| IL-IIA | Secukinumab/Cosentyx | Anti -Secukinumab | mAb, Mouse |
| IL-4Rα | Dupilumab/Dupixent | Anti-Dupilumab | mAb, Mouse |
| IL-6 receptor | Tocilizumab/Actemra | Anti-Tocilizumab | mAb, Mouse |
| IL-6 receptor | Sarilumab/Kevzara | Anti-Sarilumab | mAb, Mouse |
| RSV F | Palivizumab/Synagis | Anti-Palivizumab | mAb, Mouse |
Validation Case Study Examples
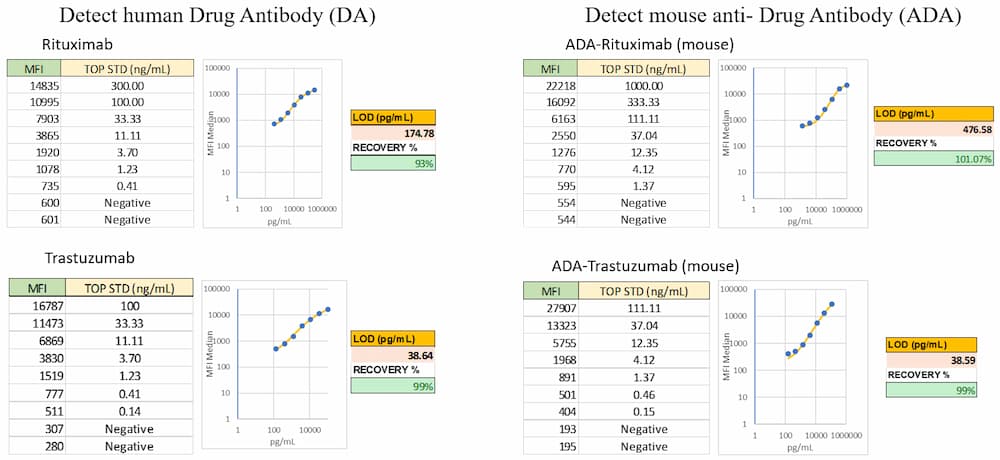
Example flow cytometry data from to detect an antibody drug (Rituximab and Trastuzumab, left), and to detect anti-drug mouse antibodies to Rituximab or Trastuzumab (right). Data includes standard curve MFI values, LOD, and percent recovery.
Discuss your project requirements
Pharmacodynamics (PD)
The paired study for pharmacokinetics is pharmacodynamics (PD). PD studies start uncovering how and why drugs interact with their organism. These studies usually revolve around either biochemical effects, or physiological effects on the organism. Depending on the drug and study, specific assays may be needed, but often include studies to evaluate receptor binding, dose-responses, and drug efficacy amongst others.
RayBiotech employs multiple flow-based models to determine the Receptor Occupancy of the drug/molecule in question. Free, Occupied, and Total Receptor levels can all be enumerated with a simple flow-based method.
Drug PD Services Available
| Testing Needs | RayBiotech Assays |
|---|---|
| Receptor binding | Receptor Occupancy (RO) Assay |
| Dose-response | Cell Toxicity Assay |
| Drug Efficacy | Cell Toxicity Assay |
Drug PD Service—Receptor Occupancy (RO) Assay Methods
- Free receptor assay — measure receptors that are not bound by the drug, by assessing a fluorophore-conjugated antibody that competes with the drug for the same epitope.
- Occupied receptor assay — measure receptors that are actively bound by the drug, by assessing a conjugated anti-human secondary antibody or anti-idiotype conjugated antibody that detects bound drug.
- Total receptor assay — measure total receptors present on the cells, by assessing a conjugated non-competitive antibody that binds to a different epitope.
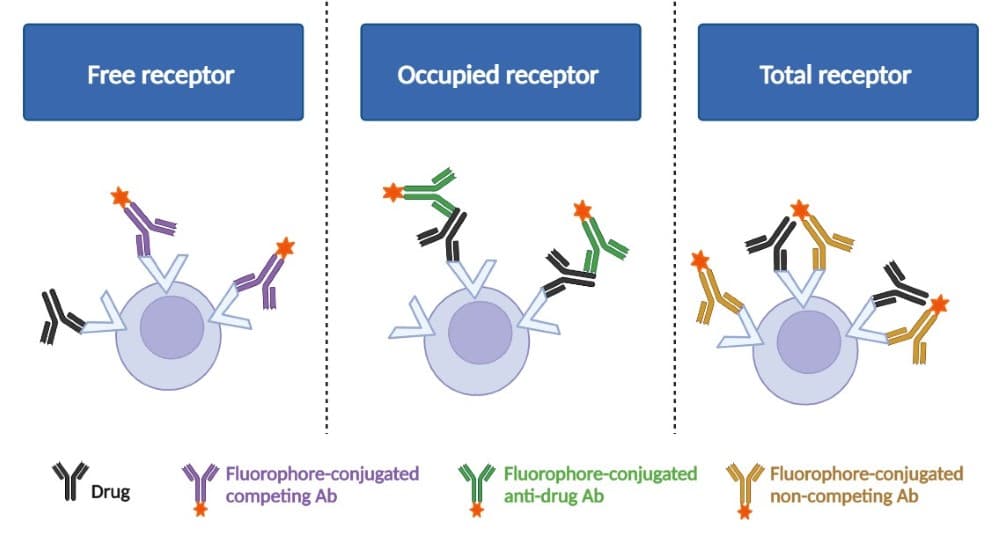
Figure 1. Schematic diagram of three basic RO assay format.
Cell Toxicity Assay for PD Studies
Cell Toxicity Assays via flow cytometry can determine the numbers and survival rate of target cells after drug treatment. A therapeutic antibody attaches itself to an antigen on the surface of the target cell, such as a cancer cell, and can serve as a flag to attract disease-fighting molecules or immune cell to promote the cells destruction.
Mechanisms for antibody mediate targeting include Antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cell-mediated phagocytosis (ADCP), both which directly target a cell for death by immune cells. On the other hand, complement-dependent cytotoxicity (CDC) is an antibody’s interaction with complement component C1q which initiates the complement cascade an indirect cell death by perforating the target itself.
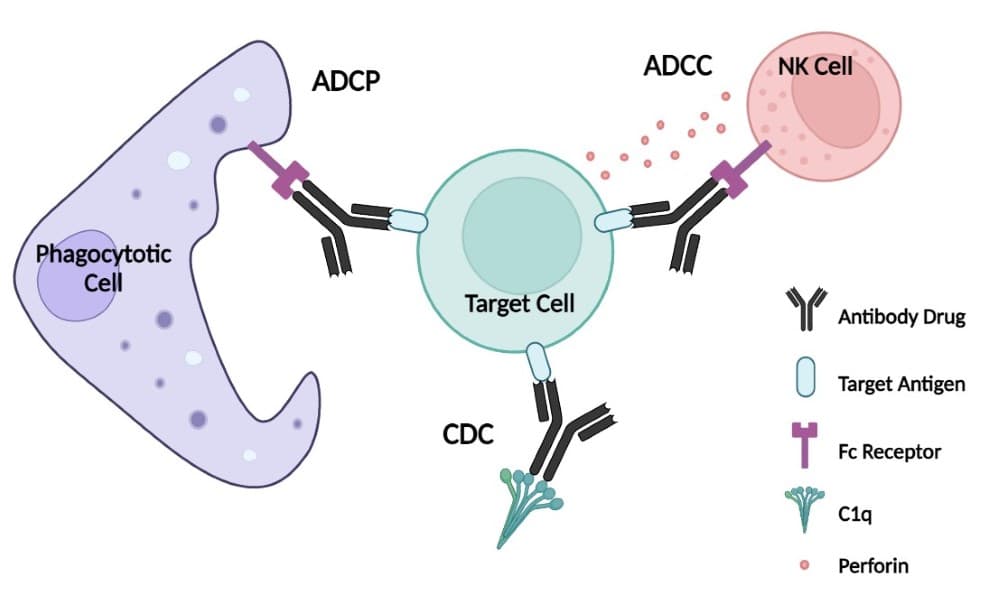
Figure 2. Mechanisms of action (MOA) of antibody-mediated effector functions. ADCC: antibody-dependent cell-mediated cytotoxicity. ADCP: antibody-dependent cell-mediated phagocytosis. CDC: complement-dependent cytotoxicity.
Receptor Occupancy Assay: Rituximab, a CD20 specific monoclonal antibody
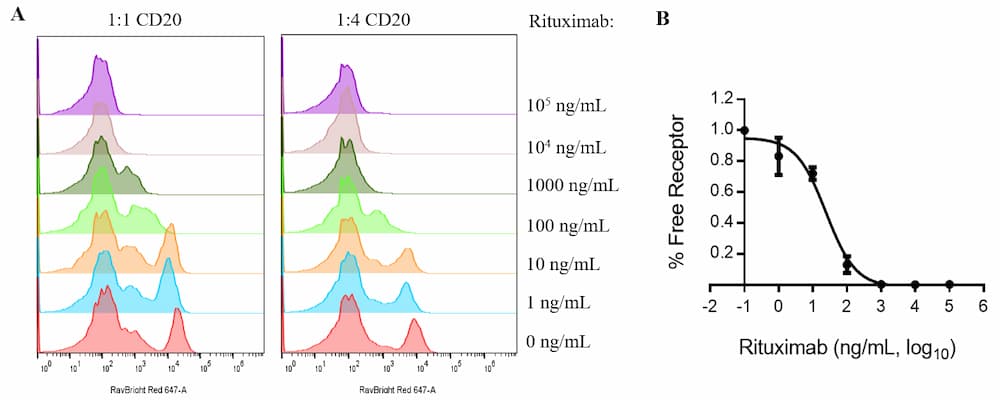
Figure 3. Receptor occupancy of Rituximab upon human PBMCs. Human PBMCs were treated with different doses of Rituximab, starting with 0 ng/mL and increasing to 100000 ng/mL. After 24 h treatment, PBMCs were stained with anti-human CD20 (Rituximab biosimilar). (A) Titration of different concentration of CD20 antibody. (B) Percentage of free receptor calculated by comparing fluorescent signal of untreated and Rituximab treated PBMCs.
Cell Toxicity Assay Case Study via ADCC : Trastuzumab a HER2 monoclonal antibody
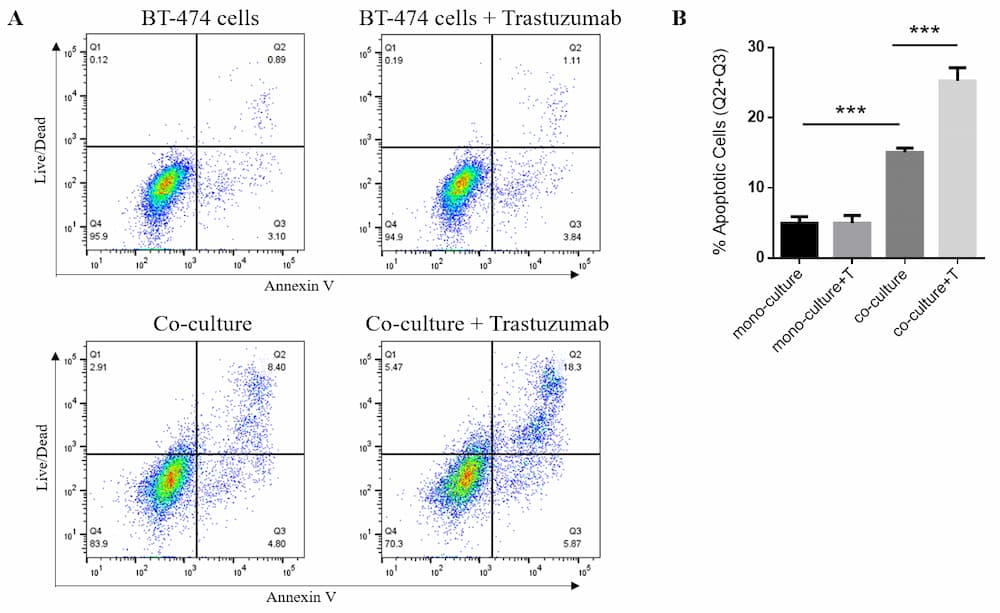
Figure 4. Flow cytometric analysis of ADCC. BT-474 breast cancer cells (target cells) were co-cultured with human PBMCs (effector cells). Cells were treated with 10ug/mL of Trastuzumab. After 24 h treatment, cells were stained with Annexin V and RayBright Live (Kit; 137-08010). (A) Dot plots of Live marker vs. Annexin V. (B) Apoptotic cells by percent positive for live/dead marker and Annexin V.