Antibody Drug Development & Characterization
RayBiotech has a comprehensive catalog of products and services to advance drug discovery, from off-the-shelf arrays to screen for drug candidates to custom assays and services. Our comprehensive analytical services ensure compliance with CLIA, good clinical laboratory practices (GCLP), good laboratory practice (GLP), and current good manufacturing practice (cGMP) standards as well as current regulatory guidance documents based on industry requirements.
Antigen, Protein, and Drug Production
Protein-based drugs are biological or biopharmaceutical drugs that are derived from proteins themselves. These molecule types include monoclonal and even polyclonal antibodies, enzymes, hormones, cytokines and vaccines. Different from small molecule drugs that are often chemically synthesized, these target molecules have a biological origin, often produced in bacteria, yeast, or mammalian cells. Biologically produced proteins have several advantages in that they can be biologically complex and can also substantially increase and improve their specificity for molecules of interest.
Since these molecules are produced in vitro, RayBiotech’s GMP-certified custom protein production encompasses every step of the process and can help produce your molecules of need. Our process involves everything from gene cloning through protein purification and includes substantial and customer driven downstream characterization and quality control.
In vitro assay and screening
A good experiment often starts in vitro where initial mechanisms, methods, and ideas are tested. We can help characterize your targets response or mechanism of action in a number of methods. Those tests can be binding specificity, binding affinity, functional properties, and immunogenicity to name a few. We employ methods like flow cytometry, sandwich and direct ELISA testing, western blotting, multiplex detection, and functional testing depending on the customers needs.
We can start the project right at the start and walk you through the proposed cell line, treatment, model of testing and sample collection. Or, if you’ve got samples ready to go and get tested, we can start from there instead. The customer drives the entire project, and we are here to help with getting it done. RayBiotech has been serving as that starting point for over 20 years, helping everyone from in silico startups to big pharma get their projects started.
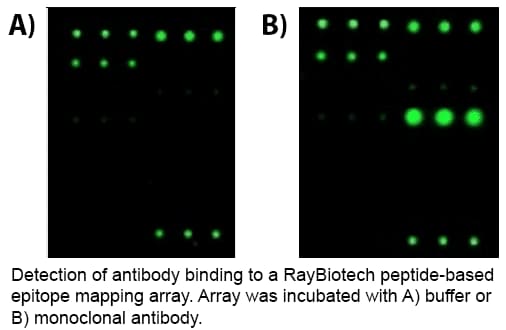
Epitope mapping
Epitope mapping is often employed to identify the antigen binding sites of an antibody. RayBiotech’s peptide-based epitope mapping arrays are prepared by first printing overlapping, chemically-synthesized peptides (> 95% purity) spanning the length of the protein-of-interest. Each peptide is printed in replicates onto a glass slide surface in an arrayed format for each overlapping peptide. Purified antibodies or bodily fluids containing antibodies incubated on the peptide arrays, during which the antibodies specifically bind to the peptides displaying their sequence-of-interest. A biotin-conjugated secondary antibody (e.g., anti-human IgG, mouse IgG) and a fluorescence dyed streptavidin molecule are added to the array for detection. Fluorescent signal is then captured, and peptide sequences that are bound can be identified and potential epitopes ascertained.
Epitope mapping is ideal for:
- Identify antigenic epitopes for antibody & vaccine development
- Locate (auto)antigen epitopes of (auto)antibodies
- Define the best antibody clone against the protein-of-interest
- Perform epitope mutant analysis
- Map B cell epitopes
Antibody Production Services
RayBiotech offers a complete antibody production line, from custom antibody production services including polyclonal and monoclonal developments, to bulk scale production of customer needed clones in house, and purification or labeling to customer required fluorescent dyes. We are happy to help wherever and however your antibody needs come from.
Custom antibody development covers every step of monoclonal and polyclonal development, and includes antigen design (including phospho), synthesis and carrier protein conjugation, immunization, titer analysis, serum collection or hybridoma fusion, and antibody purification.
Other antibody production services includes bulk scale up production of your monoclonal antibodies, and we can use a carrier free system, or serum containing system, perform endotoxin testing, purification, isotyping characterization, neutralization assessment, and more. After production, or if a client needs, we also offer other custom options as well including antibody conjugation, lyophilization, custom vialing & aliquoting, and antibody validation services by Western blot, ELISA, or immunoprecipitation.
Pharmacokinetics and Pharmacodynamics
Pharmacokinetics (PK) studies of any drug revolve around how the organism affects the drug, while pharmacodynamics (PD) studies work to uncover how and why drugs interact with their organism.
For PK, RayBiotech offers many options to test in vitro samples, as well as serum and plasma drug levels in your samples to measure the interactions ongoing. Our ELISA Drug antibody and anti-drug antibody ELISA and RayPlex kits are available for many widely tested drugs. Or for PD studies, RayBiotech employs multiple flow-based models to determine the Receptor Occupancy of the drug/molecule in question. Free, Occupied, and Total Receptor levels can all be enumerated with a simple flow-based method. Additionally, RayBiotech offers specially designed cell toxicity assays to determine drug dose responses and drug efficacy testing in vitro.
Our PK/PD team is here to help you design your assay of need, and engineer a solution to provide you the data to move your project forward. Learn more from our case studies and assay options on PK and PD Here: