RayBio® Human Osteopontin (SPP1) ELISA Kit for cell culture supernatants, plasma, and serum samples.
Lead time: Typically ships within 1-2 business days. No Friday shipments.
Product Description
Specifications
| Size | 1 Plate Kit, 2 Plate Kit, 5 Plate Kit |
|---|---|
| Species | Human |
| Accession Number | P10451 |
| Gene Id | 6696 |
| Gene Symbols | SPP1|BNSP|OPN|PSEC0156 |
| Protein Name / Synonyms | Osteopontin (Bone sialoprotein 1) (Nephropontin) (Secreted phosphoprotein 1) (SPP-1) (Urinary stone protein) (Uropontin) |
| Quantitative/Semi-Quantitative | Quantitative |
| Specificity | This ELISA kit shows no cross-reactivity with any of the following cytokines tested (human Angiogenin, BDNF, BLC, ENA-78, FGF- 4, IL-1 alpha, IL-1 beta, IL-2, IL-3, IL-4, IL-5, IL-7, IL-8, IL-9, IL-10, IL-11, IL-12 p70, IL-12 p40, IL-13, IL-15, I-309, IP-10, G-CSF, GM-CSF, IFN-gamma, Leptin, MCP-1, MCP-2, MCP-3, MDC, MIP-1 alpha, MIP-1 beta, MIP-1 delta, PARC, PDGF, RANTES, SCF, TARC, TGF-beta, TIMP-1, TIMP-2, TNF-alpha, TNF-beta, TPO, VEGF. |
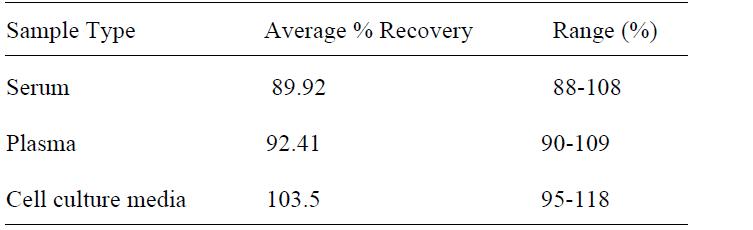
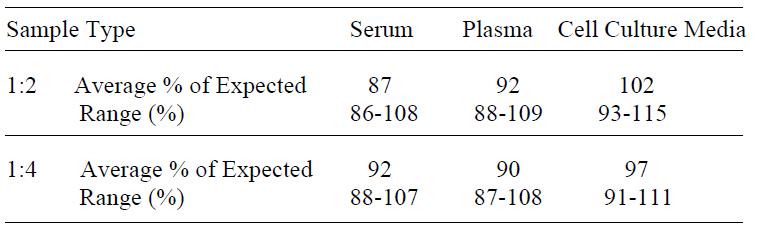
| Compatible Sample Types | Cell Culture Supernatants, Plasma, Serum |
| Solid Support | 96-well Microplate |
| Method Of Detection | Colorimetric |
| Design Principle | Sandwich-based |
| Sensitivity | 50 pg/ml Need more sensitivity? Check out the new BIQ-ELISA™ kit for this target. Still not enough? Then your answer is our Ultrasensitive Biomarker Testing Service powered by Simoa™ technology. |
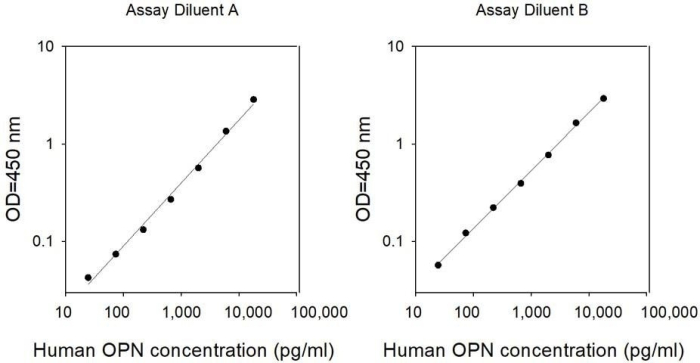
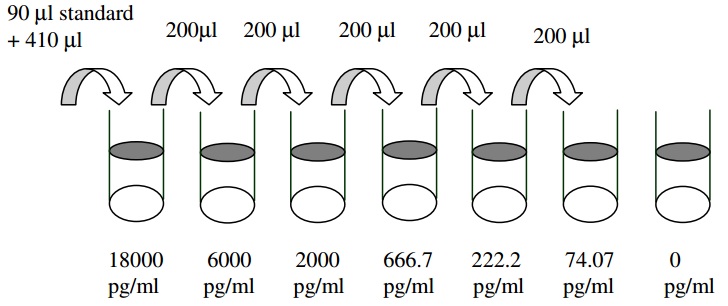
| Detection Range | 50 pg/ml - 18000 pg/ml |
| Recommended Dilution (Serum/Plasma) | 3 - 100 fold |
| Estimated Lead Time | 1-2 business days |
| Shipping Type | Blue ice |
| Storage | ≤-20°C |
Risk-Free Guarantee
We offer a 100% guarantee on all ELISA kits and membrane cytokine arrays.
Learn More
Amazon Gift Cards!
$5 Amazon gift card in every kit box purchased.
Wu T., Ding H., Han J., et al. Antibody-Array-Based Proteomic Screening of Serum Markers in Systemic Lupus Erythematosus: A Discovery Study. J Proteome Res. 2016 Jul 1;15(7):2102-14. doi: 10.1021/acs.jproteome.5b00905.
Nagy E, Eriksson P, Yousry M, et al. Valvular osteoclasts in calcification and aortic valve stenosis severity. Int J Cardiol. 2013 Feb 27; [Epub ahead of print] doi:10.1016/j.ijcard.2013.01.207.
Bassyouni IH, El-Wakd MM, Bassyouni RH. Soluble levels of osteopontin in patients with Behcet's disease: association with disease activity and vascular involvement. J Clin Immunol. 2013 Feb;33(2):361-367.
Bassyouni I., Bassyouni R., Ibrahim N., Soliman A. Elevated serum osteopontin levels in chronic hepatitis C virus infection: association with autoimmune rheumatologic manifestations. J Clin Immunol. 2012 Dec;32(6):1262-9. doi: 10.1007/s10875-012-9727-7.
Von Neuhoff N, Oumeraci T, Wolf T, et al. Monitoring CSF Proteome Alterations in Amyotrophic Lateral Sclerosis: Obstacles and Perspectives in Translating a Novel Marker Panel to the Clinic. Borchelt DR, ed. PLoS ONE. 2012;7(9):e44401. doi:10.1371/journal.pone.0044401.
Taranta-Janusz K., Wasilewska A., Stypulkowska J., Sutula M. Osteopontin and symmetric dimethylarginine plasma levels in solitary functioning kidney in children. Acta Paediatr. 2012 Aug;101(8):e369-72. doi: 10.1111/j.1651-2227.2012.02690.x
Gluba-Brzozka A., et al. Markers of increased cardiovascular risk in patients with chronic kidney disease. Lipids in Health and Disease 2014, 13:135 doi:10.1186/1476-511X-13-135
Erturkler, Enis, et al. "Plasma osteopontin levels in patients with Behcet's disease and psoriasis." European Journal of Dermatology 21.2 (2011): 203-208.
Shaker, Olfat, et al. "Osteopontin gene polymorphisms as predictors for the efficacy of interferon therapy in chronic hepatitis C Egyptian patients with genotype 4." Cell biochemistry and function 31.7 (2013): 620-625.
Acar A., Cevik M., Arikanoglu A., et al. Serum levels of calcification inhibitors in patients with intracerebral hemorrhage. Int J Neurosci. 2012 May;122(5):227-32. doi: 10.3109/00207454.2011.642039.
-
OsteopontinWe ran 3-D cell culture supernatants were run with the kit for the first time as feasibility. We had two different media formulations as background against the culture conditions over a month long culture period. A 3x dilution worked adequately.
from Miromatrix,
on
ELISA for human PBMCWe used this kit to detect OPN production levels after stimulating human PBMCs with LPS. OPN production was well detected in a two-fold-diluted culture medium.from duke,
on
ELISA for pancreatic plasma samplesWe used this kit for OPN detection in plasma samples. Overall, the calibration and sensitivity of the assay meet the expectations. However, we had to use low diluted plasma samples to be able to detect levels of OPN. So, dilution optimization will be most likely required before running higher number of samples.from OHSU,
on
Write Your Own Review