ELISA Kit Categories
Popular ELISA Kits
New Products
What customers are saying
Rigorous Testing for Quality You Can Trust
At RayBiotech, we take our product quality very seriously. We apply a battery of rigorous product-specific quality control tests to ensure your experiments are set up to produce high-quality results.
Reproducibility and Precision
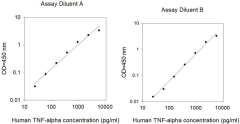
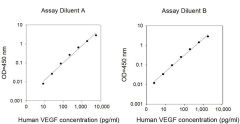
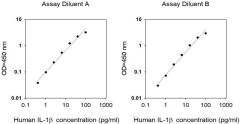
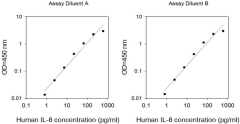
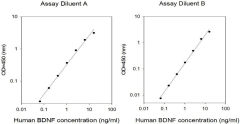
We evaluate intra-assay reproducibility on sandwich ELISA kits by running 2-3 positive control samples in duplicate on a single plate (maximum tolerance = 10% CV). We use 2-3 positive control samples and a full standard curve (maximum tolerance = 12% CV) in at least 2 independent experiments. Our lot-to-lot consistency is tested by comparing calculated concentrations of the 2-3 positive control samples with calibration curves of the current lot and previous lot (maximum tolerance = 20% CV).
Dilution Linearity and Recovery
We determine recovery by spiking various levels of target protein into biological samples. Our dilution linearity is tested by performing 2-fold and 4-fold dilutions. Recoveries typically range from 80-130% (maximum tolerance 70-150%). The biological samples tested include serum, EDTA plasma, citrate plasma, heparin plasma (normal healthy donors), and cell culture medium (DMEM or 1640). This testing series determines our recommended dilution ranges for serum and plasma. For lysate-specific sandwich ELISAs (catalog numbers ending in “-CL”), sample types include cell lysates and tissue homogenates of various origins.
Four Steps to Antigen Analysis
Each type of ELISA works a little differently, but they all follow the same basic process:
Plate coating
First, antigens are directly or indirectly immobilized on the surface of micropates.
Plate Blocking
Next, a blocking buffer containing an irrelevant protein is added to bind to the remaining surfaces.
Antibody Incubation
Then, an antigen-specific antibody is added. It binds to the antigens during the incubation step.
Detection
Finally, the signal generated by the direct or secondary tag on the antibody is detected and measured.
Frequently Asked Questions
Still have questions?
Still have questions?